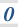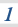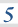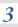3.1.10 Equilibrium constant, Kp, for homogeneous systems
The further study of equilibria considers how the mathematical expression for the equilibrium constant K p enables us to calculate how an equilibrium yield will be influenced by the partial pressures of reactants and products. This has important consequences for many industrial processes.