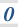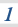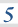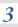3.1.6 Chemical equilibria, Le Chatelier’s principle and Kc
In contrast with kinetics, which is a study of how quickly reactions occur, a study of equilibria indicates how far reactions will go. Le Chatelier’s principle can be used to predict the effects of changes in temperature, pressure and concentration on the yield of a reversible reaction. This has important consequences for many industrial processes. The further study of the equilibrium constant, Kc , considers how the mathematical expression for the equilibrium constant enables us to calculate how an equilibrium yield will be influenced by the concentration of reactants and products.